ObvioGo®: Powering the Future of Clinical Trials
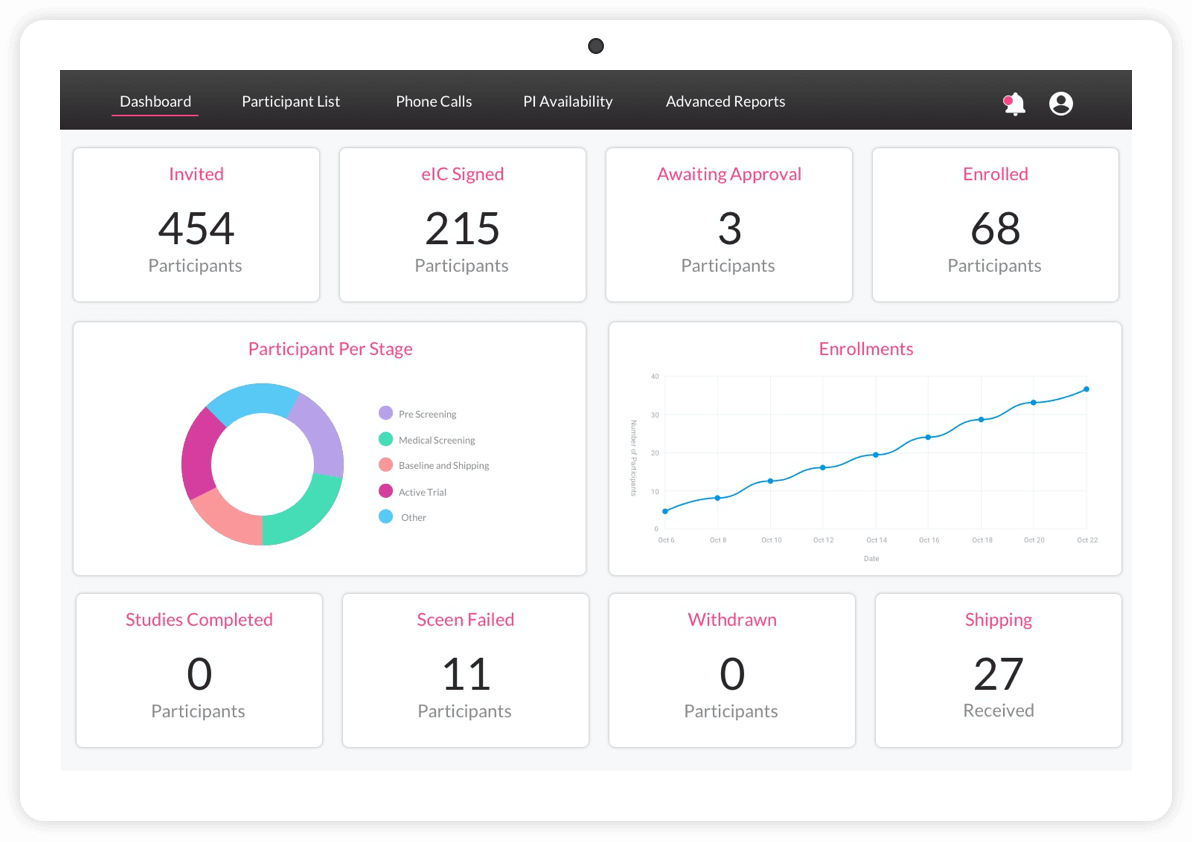
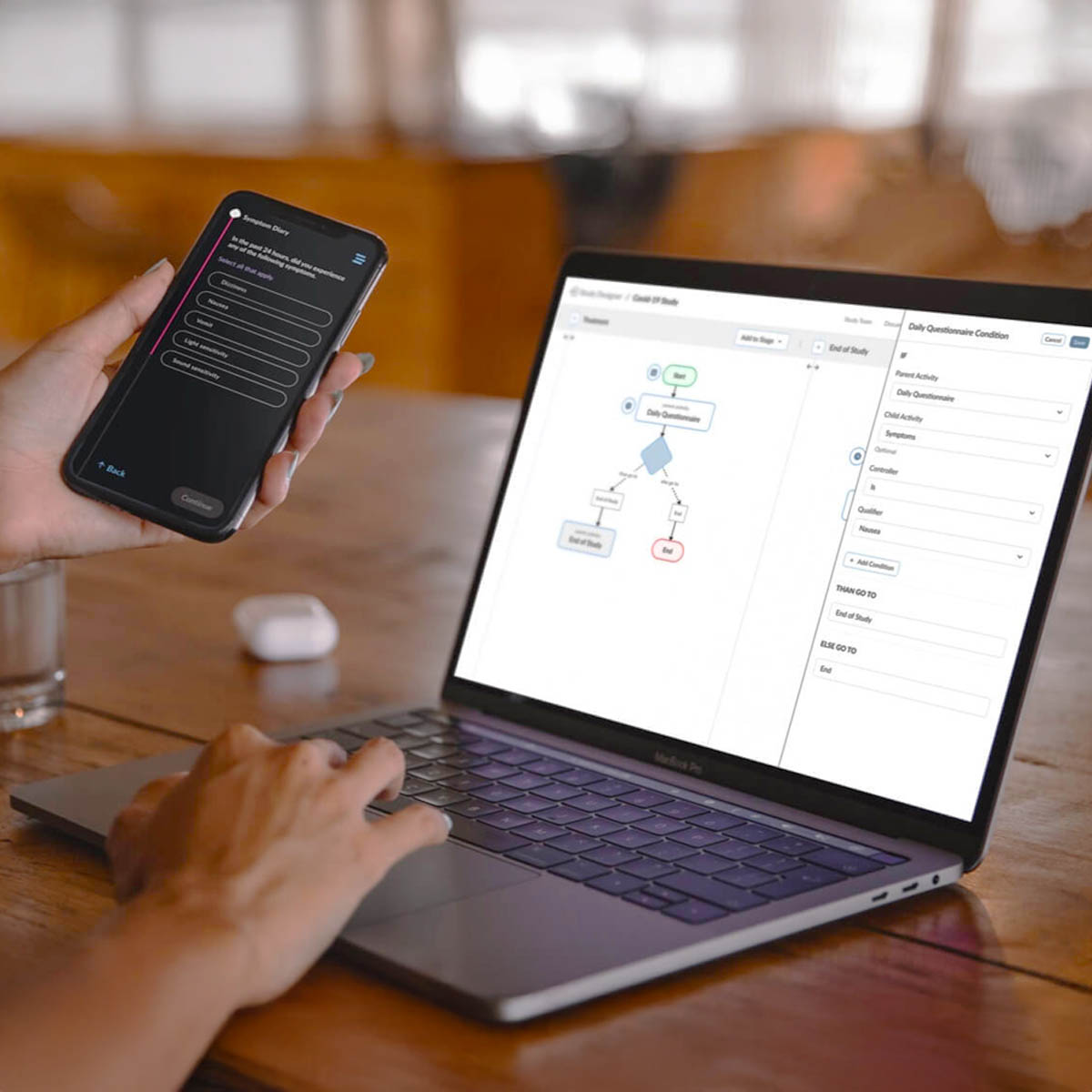
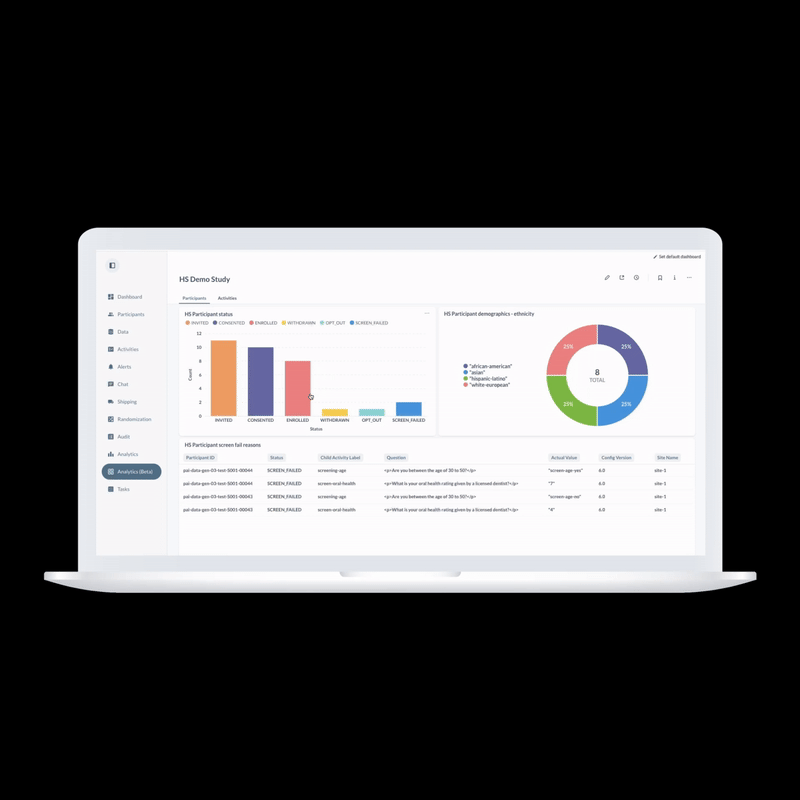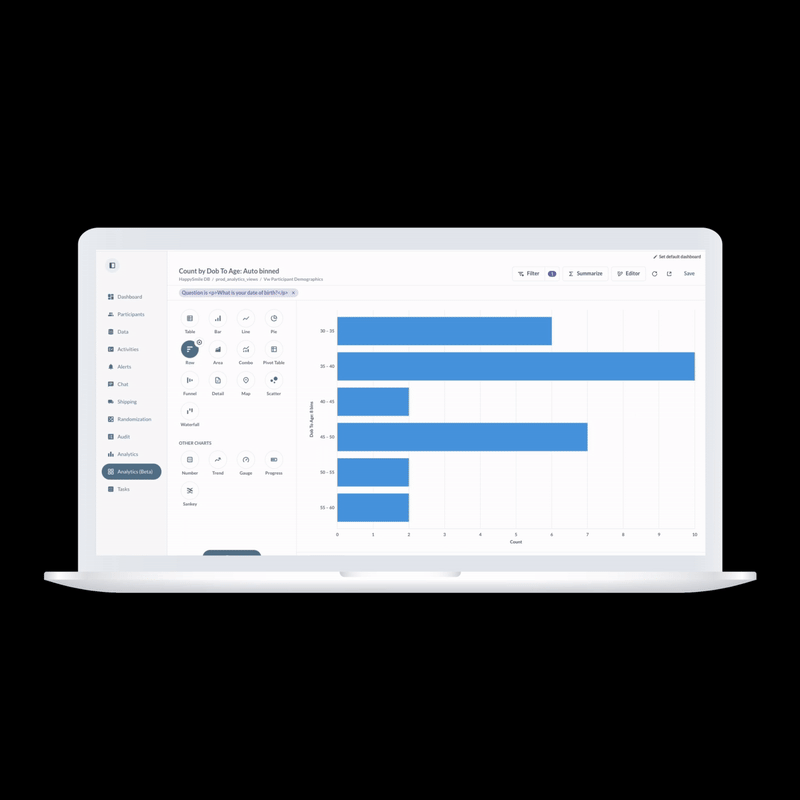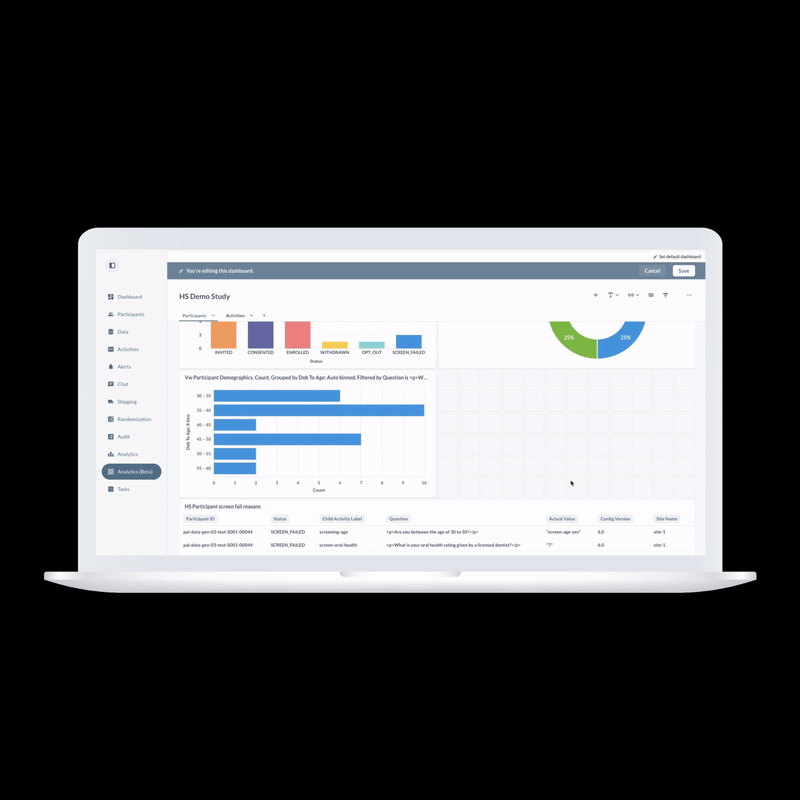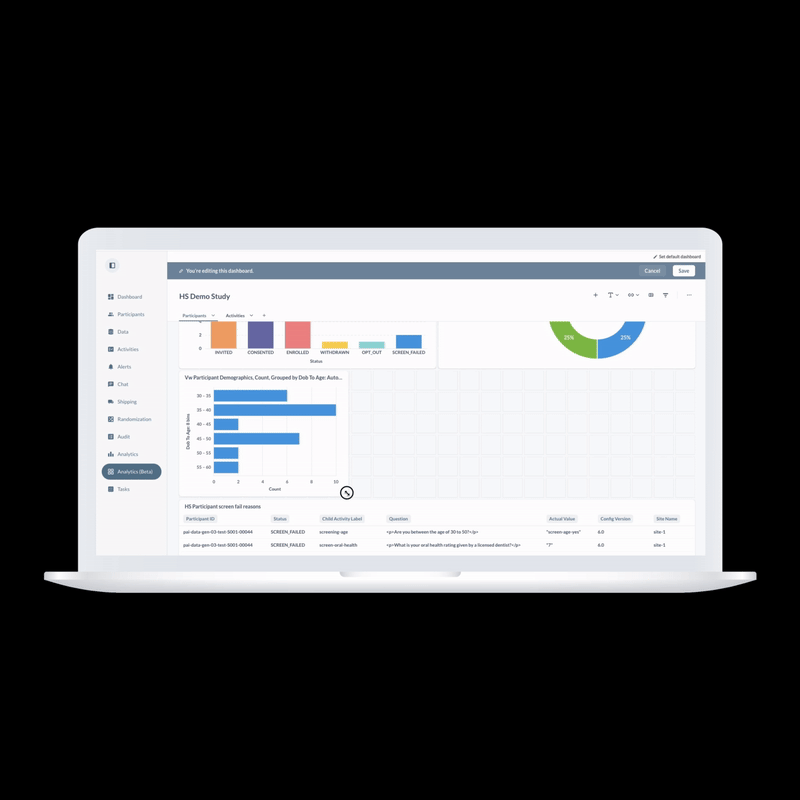
ObvioHealth’s next-generation platform simplifies study design, boosts participant engagement, and delivers cleaner, faster data—enabling more agile and scalable decentralized and hybrid trials. Experience digital clinical trials reimagined: smarter, faster, and built for today’s demands.
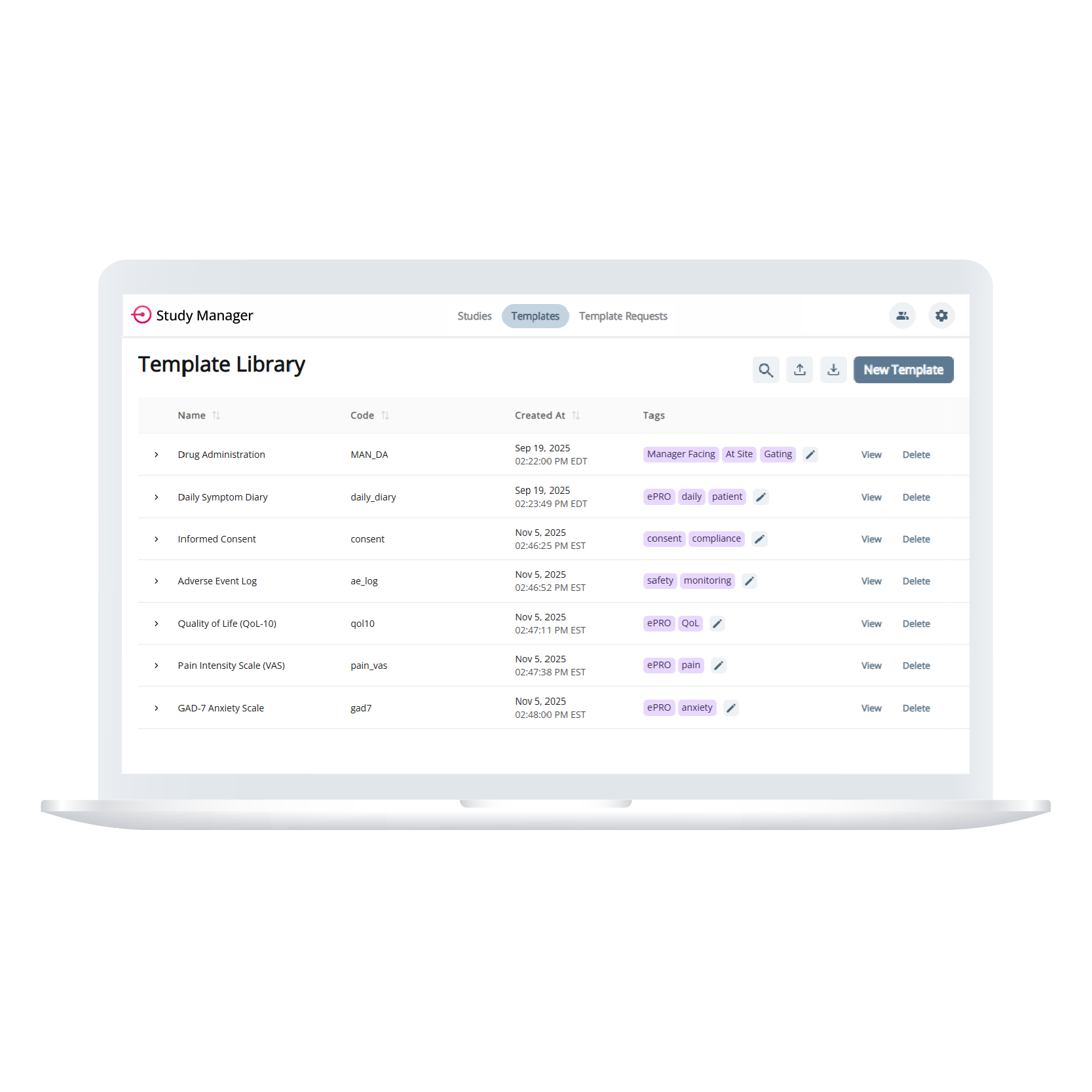
Template Library