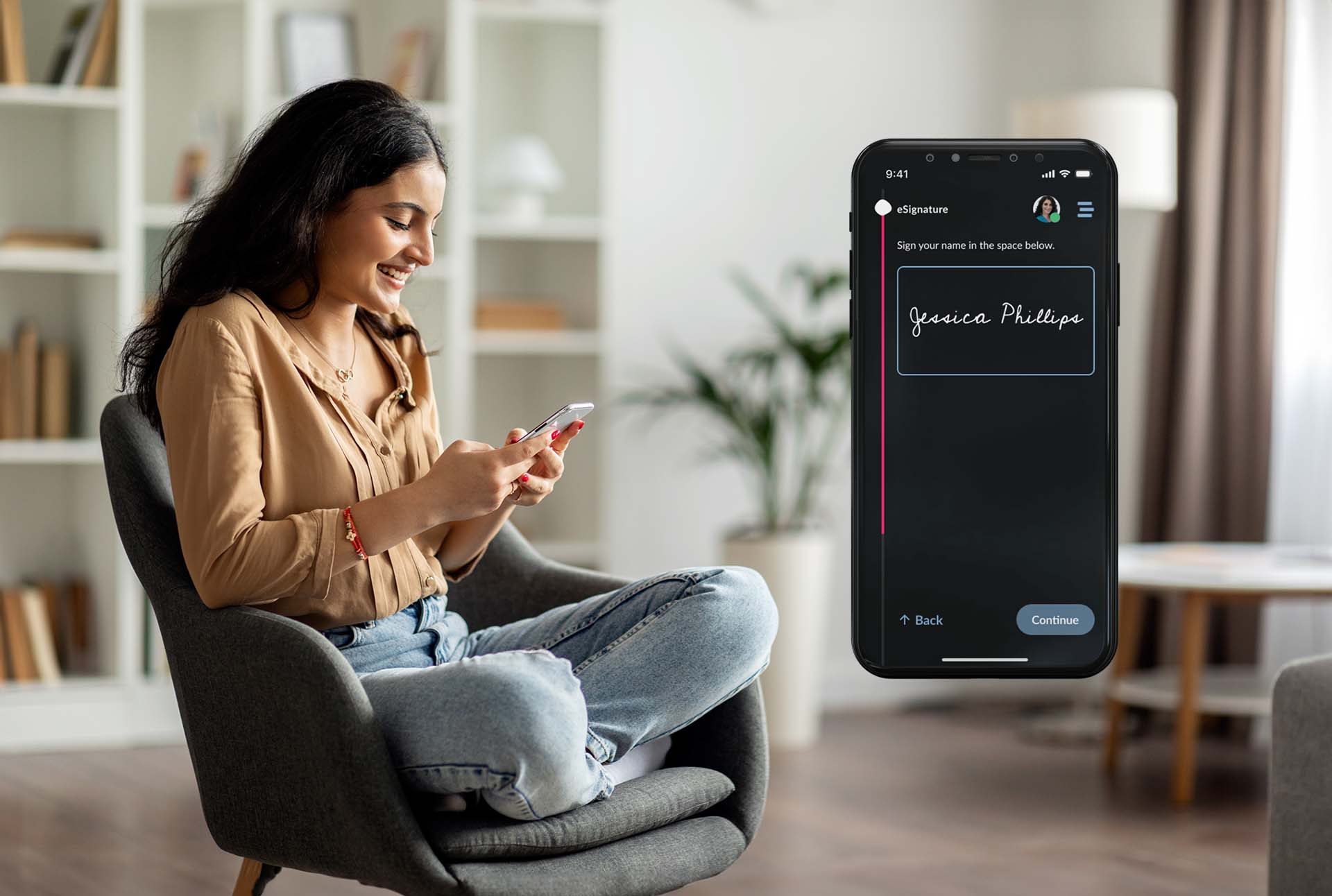
Step 3:
eConsent & Enrollment
Smarter. Simpler. Fully Compliant.
ObvioHealth’s eConsent and enrollment tools set clinical trials on the path to success—right from the start. By combining in-house technology, participant-centric design, and seamless integration, we reduce site burden, improve regulatory compliance, and enhance participant understanding and retention.