Go Beyond the Site. Go with ObvioGo.
ObvioGo gives pharma and biotech teams the tools to run faster, more flexible digital-first trials—generating stronger evidence and submission-ready results with less operational burden.

ObvioGo gives pharma and biotech teams the tools to run faster, more flexible digital-first trials—generating stronger evidence and submission-ready results with less operational burden.

Biotech and pharma sponsors face growing pressure—tight budgets, complex global regulations, and the demand for faster, high-quality evidence. ObvioGo meets these challenges with agile, efficient, and patient-centric trial delivery. From early proof-of-concept to pivotal and post-market studies, ObvioGo reduces site burden, enhances data quality, and accelerates timelines.
In a Norovirus vaccine study, our digital platform achieved 100% daily diary completion—enabling real-time safety alerts, streamlined remote monitoring, and database lock just 12 days after LPO. The result: cleaner data, faster insights, and stronger oversight.
In a fully virtual Phase IV ADHD study, ObvioGo captured safety and efficacy data from adults—many with co-morbid symptoms and concurrent medications. Our platform enabled inclusive, remote monitoring through ePROs, eClinROs, and connected devices—generating real-world outcomes with regulatory-grade precision.
In an Acute Pain Study, 603 patients were enrolled across four APAC countries. The hybrid design and real-time ePRO capture delivered 99% retention and 90% medication adherence—driving strong engagement and demonstrating operational success in diverse care settings.

"ObvioHealth’s platform is really powerful in providing safety oversight. The real-time alerts enabled me to review vaccine data daily to report to senior management. The team was professional and very responsive to requests."
— Director, Clinical Operations, Vaxart




Clinical development averages over a decade, with long delays between phases. ObvioGo shortens startup with no-code protocol design, fast configuration, and real-time data access—helping sponsors reduce inter-trial gaps and speed time to milestones.

ObvioGo supports eConsent and ePRO in multiple languages and is built to meet global compliance standards. Whether your trial is hybrid or fully decentralized, our platform adapts to your study locations—removing geographic barriers without adding complexity.

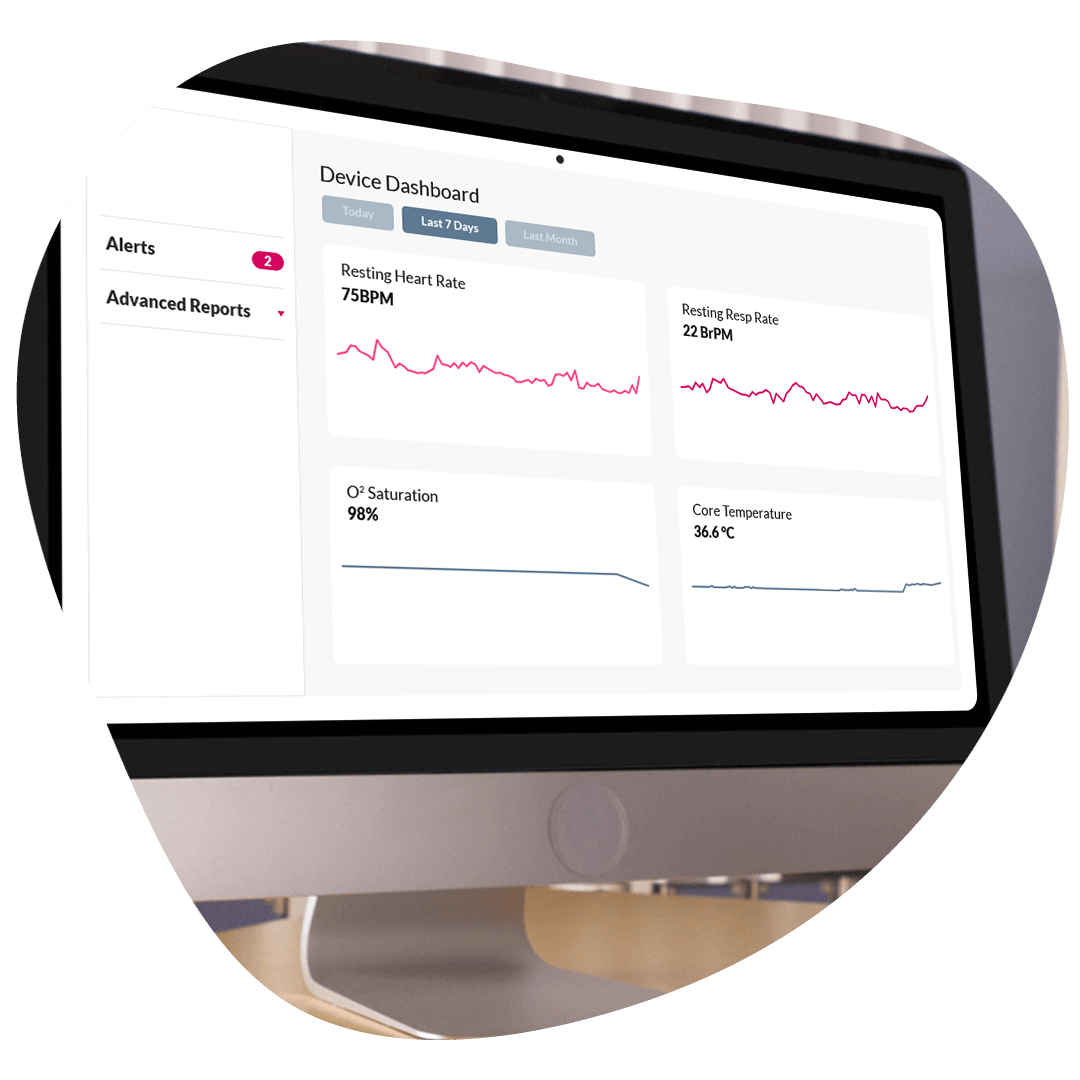
ObvioGo delivers real-time insights through customizable dashboards, layered queries, and role-based visualizations—giving sponsors full visibility into trial progress, site activity, and data quality. Structured outputs drive faster decisions and stronger outcomes.

Designed for functional service models, ObvioGo offers plug-and-play modules and scalable licensing—so you can start small and expand as your study grows. Built-in tools support sponsor oversight, CRO collaboration, and mid-study pivots without disrupting operations.

ObvioGo is designed to integrate with major EDCs, CTMS, and analytics platforms. Our architecture enables tailored integrations in partnership with study sponsors—offering centralized oversight without the need for a full system overhaul.
Whether you're a biotech launching your first study or a global pharma scaling a Phase III trial, ObvioGo flexes to fit your needs. Sponsors use ObvioGo to reduce burden, improve retention, and speed timelines—while delivering high-quality data.
We support the full study lifecycle:
From concept to completion, ObvioGo helps teams run more efficient, patient-centric trials—start to finish.
