Accelerate your Trials with ObvioGo
ObvioGo helps MedTech and DTx sponsors collect regulatory-grade data, validate therapeutic impact, and get to market faster.
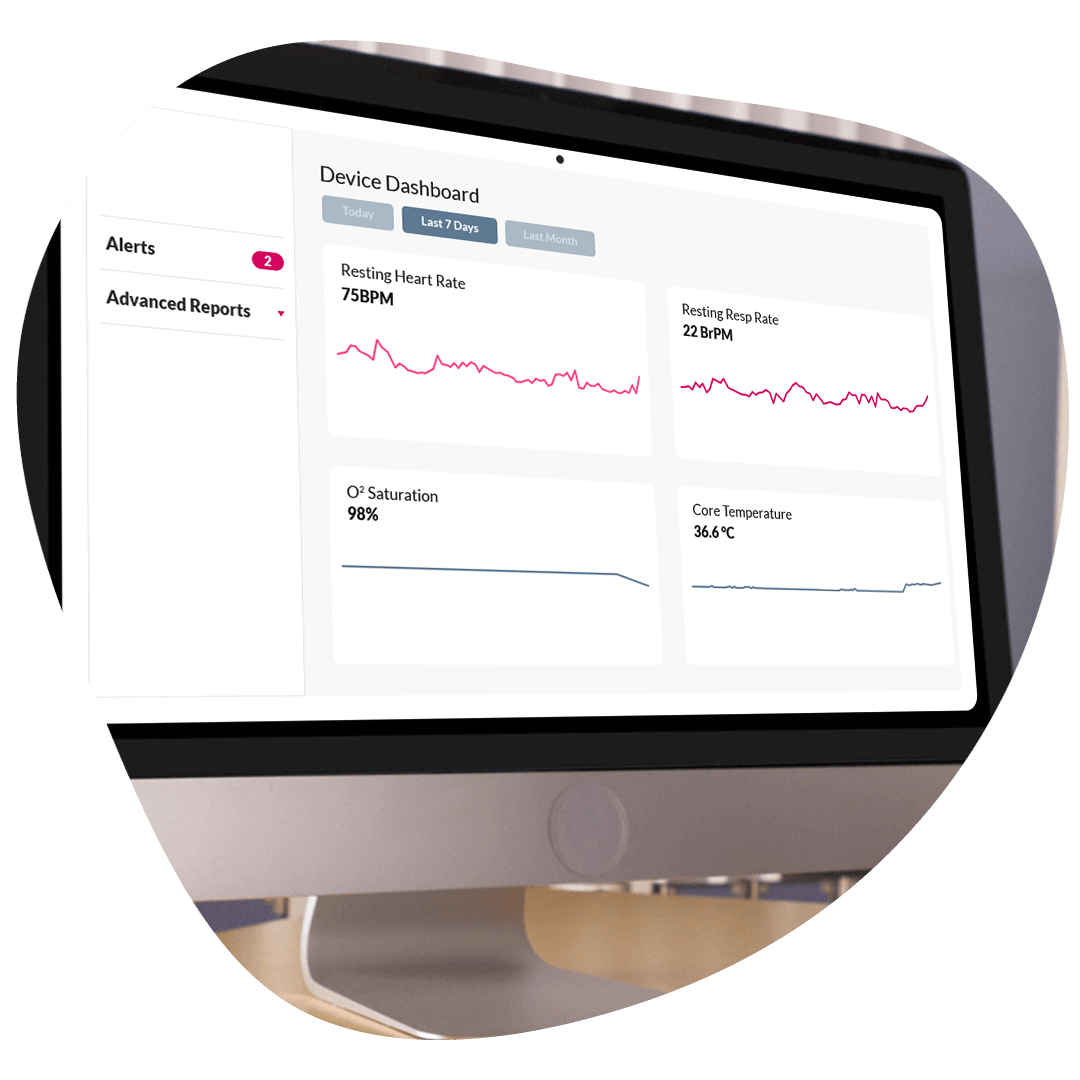
ObvioGo helps MedTech and DTx sponsors collect regulatory-grade data, validate therapeutic impact, and get to market faster.

MedTech and DTx innovators need faster, more flexible trials to keep pace with evolving regulatory standards and rapid digital product cycles. ObvioGo powers decentralized studies that validate therapeutic impact, monitor real-world adherence, and generate submission-ready and real-world evidence—on time and on budget.
In a fully virtual device study, ObvioHealth recruited 350 participants in 14 weeks and achieved 93.5% ePRO compliance and 89% retention—delivering high-quality, regulatory-grade data in the industry’s first fully virtual urogynecology trial.
Our API-first design integrates directly with therapeutic apps to capture real-time adherence and outcomes—without patient burden or manual input. Synced for regulatory-grade precision, it accelerates validation and submission timelines.
In a nationwide migraine trial, ObvioHealth recruited in just 45 days—cutting the sponsor’s expected 1.5-year timeline by over 90%. Our mobile-first platform enabled fast screening, remote onboarding, and in-app support to drive engagement at scale.

"ObvioHealth helped us figure out how to keep patients engaged without giving them more migraines. Incorporating the live chat feature and notifications into the project has eased the connection for the participants."
— Steve Schaefer CEO, Mi-Helper




Accelerate study startup with drag-and-drop design tools, remote onboarding, and eConsent. ObvioGo enables rapid protocol changes—minimizing site disruption and keeping trials on track for faster validation and submission.

Seamlessly integrate RPM devices, wearables, and DTx tools. ObvioGo captures objective, real-time data with automated ingestion, adherence tracking, and built-in alerts, supporting accurate endpoints and stronger submissions.

Ensure FDA- and EMA-aligned compliance with secure, real-time data collection. ObvioGo captures validated eSource, can integrate across EMRs and lab systems, and provides audit-ready outputs for confident regulatory submissions.

Reduce site burden and increase reach with home-based, mobile-first study models. ObvioGo supports remote enrollment, in-app reminders, and telehealth—enhancing engagement and retention across diverse patient populations.

We embed into your trial ecosystem—not just as a vendor, but as a true partner. From setup to scale, our enablement team delivers expert guidance, tech support, and training to ensure operational success from day one.
Our digital-first approach empowers medical device and DTx companies to run faster, smarter trials while improving patient outcomes and data accuracy.
ObvioHealth has successfully supported device and DTx trials across a range of indications — from women’s health to neurological conditions — delivering increased patient engagement through user-friendly remote platforms, improved data accuracy with real-time device and ePRO integration, and higher study retention rates in fully virtual and hybrid trial designs.
