ObvioGo Mobile App
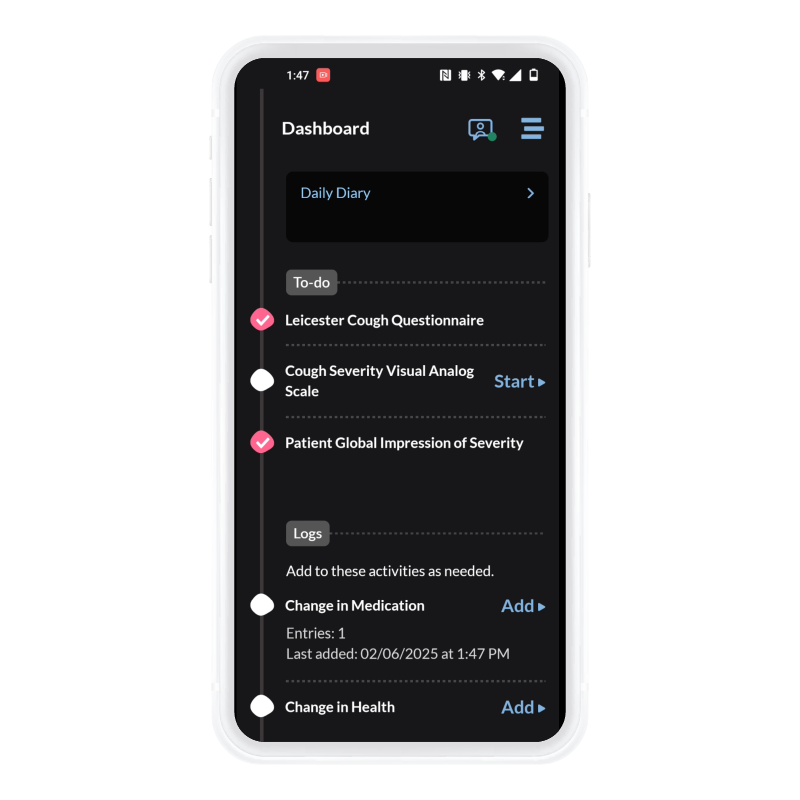
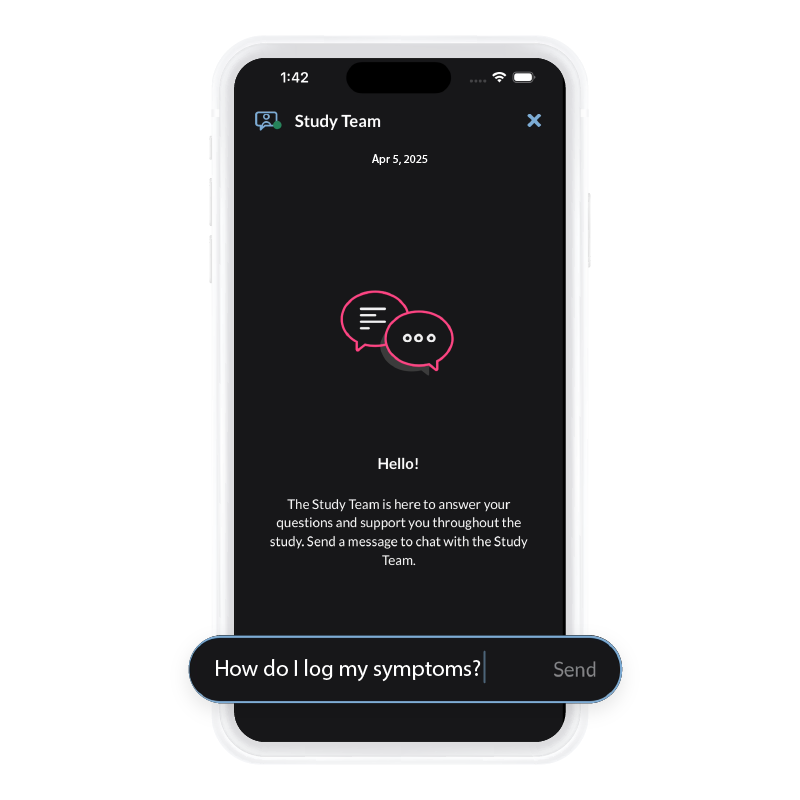
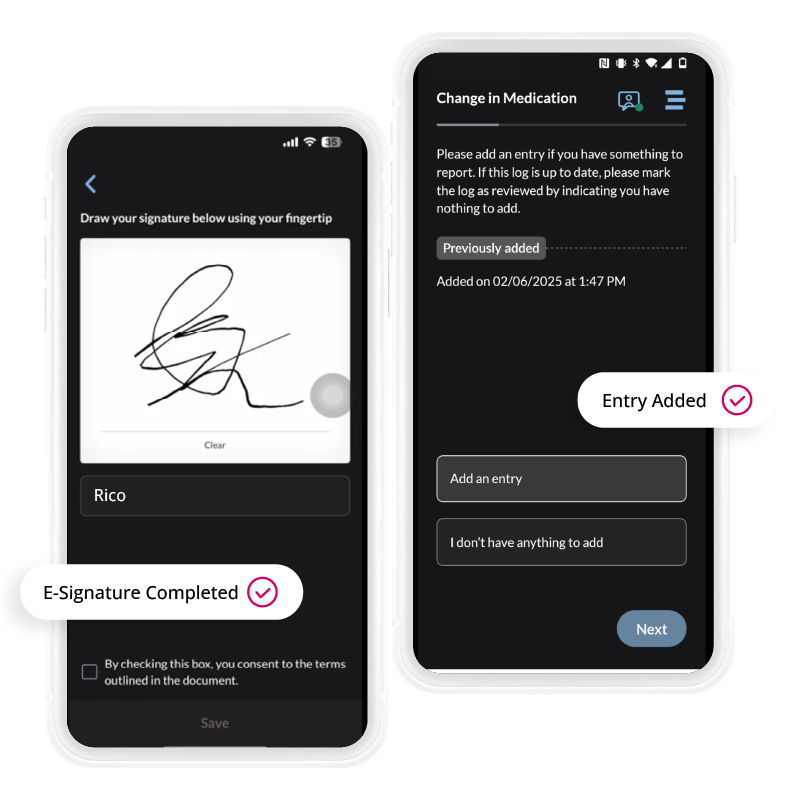
The ObvioGo App powers patient-centric trials with a secure, intuitive mobile interface—streamlining ePRO, eConsent, symptom capture, and two-way communication. Unlock real-time data, accelerate decision-making, and drive compliance through seamless remote engagement.