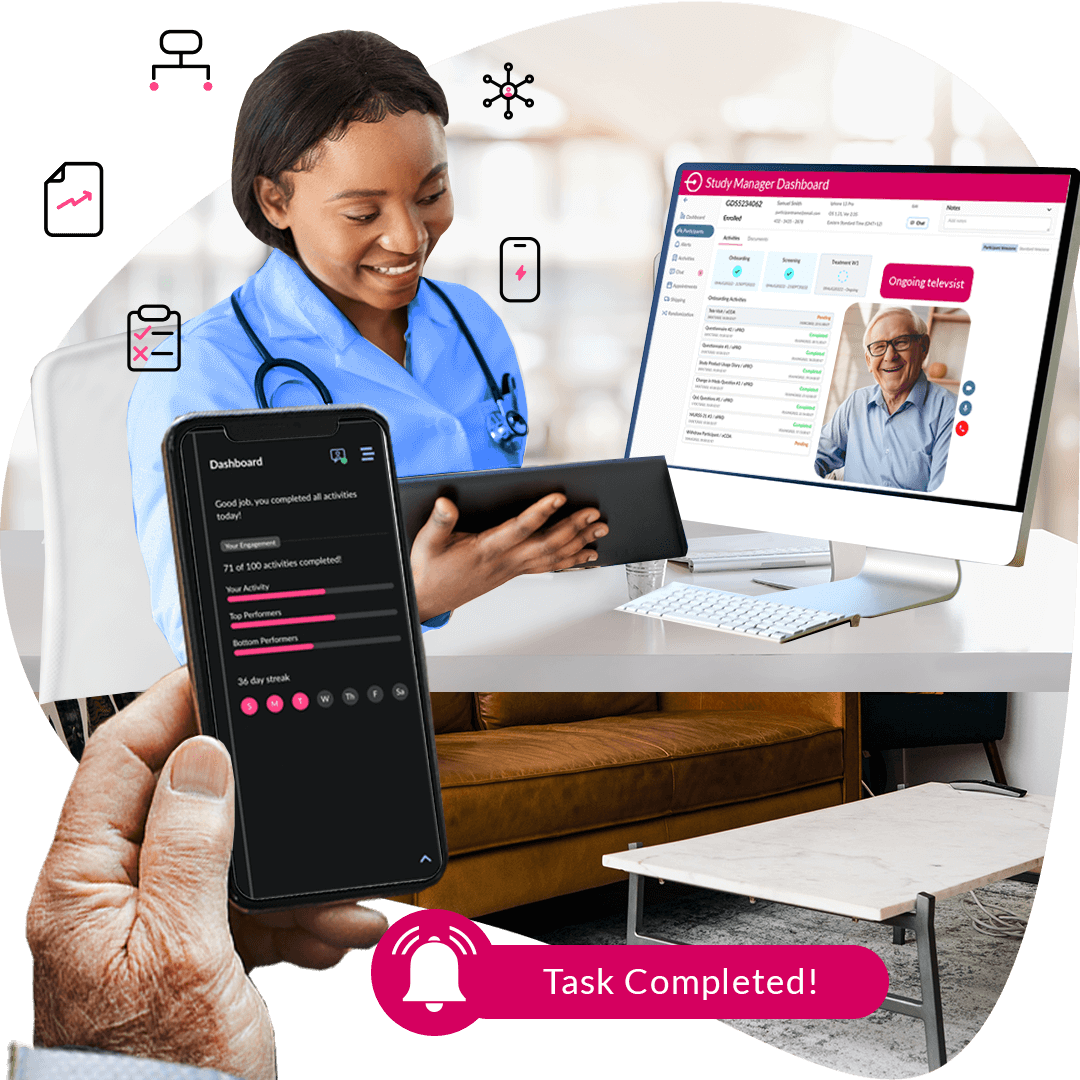
Step 7:
Virtual Visits & Support
Bringing real-time support and trial visits directly to patients—anytime, anywhere.
Un ensayo clínico es tan valioso como la fiabilidad de sus resultados. Los expertos en DCT de ObvioHealth saben cómo diseñar un ensayo para reclutar y capacitar a la cohorte óptima y eliminar el «ruido» estadístico, de modo que puedas obtener las pruebas sólidas que necesitas.