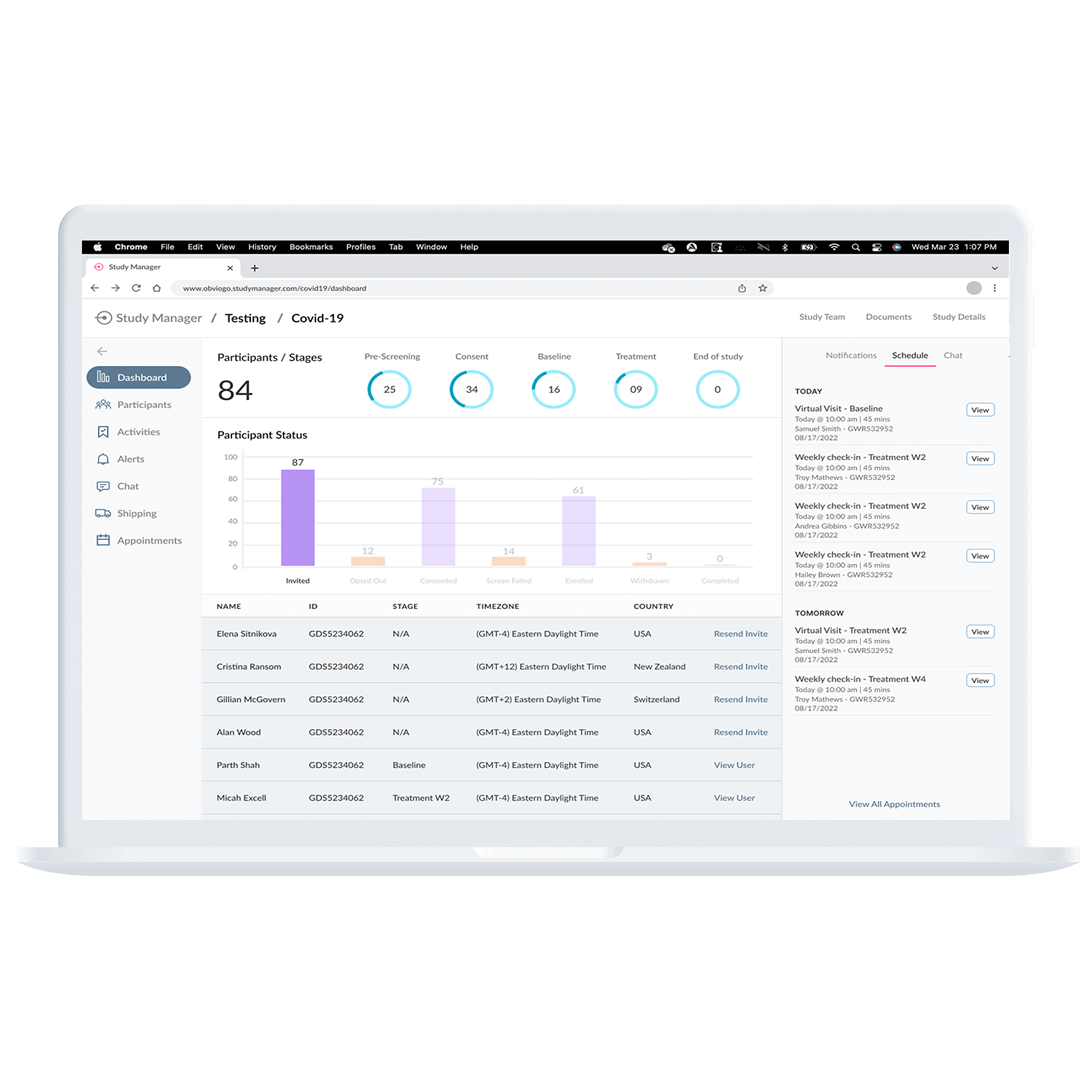
Step 6:
ePRO + eCOA
Smarter Digital Endpoints for Stronger Evidence
Un ensayo clínico es tan valioso como la fiabilidad de sus resultados. Los expertos en DCT de ObvioHealth saben cómo diseñar un ensayo para reclutar y capacitar a la cohorte óptima y eliminar el «ruido» estadístico, de modo que puedas obtener las pruebas sólidas que necesitas.