Study Manager
Designed to bring structure and speed to every phase of your trial, Study Manager combines clarity, automation, and oversight in a single solution built for modern clinical teams.
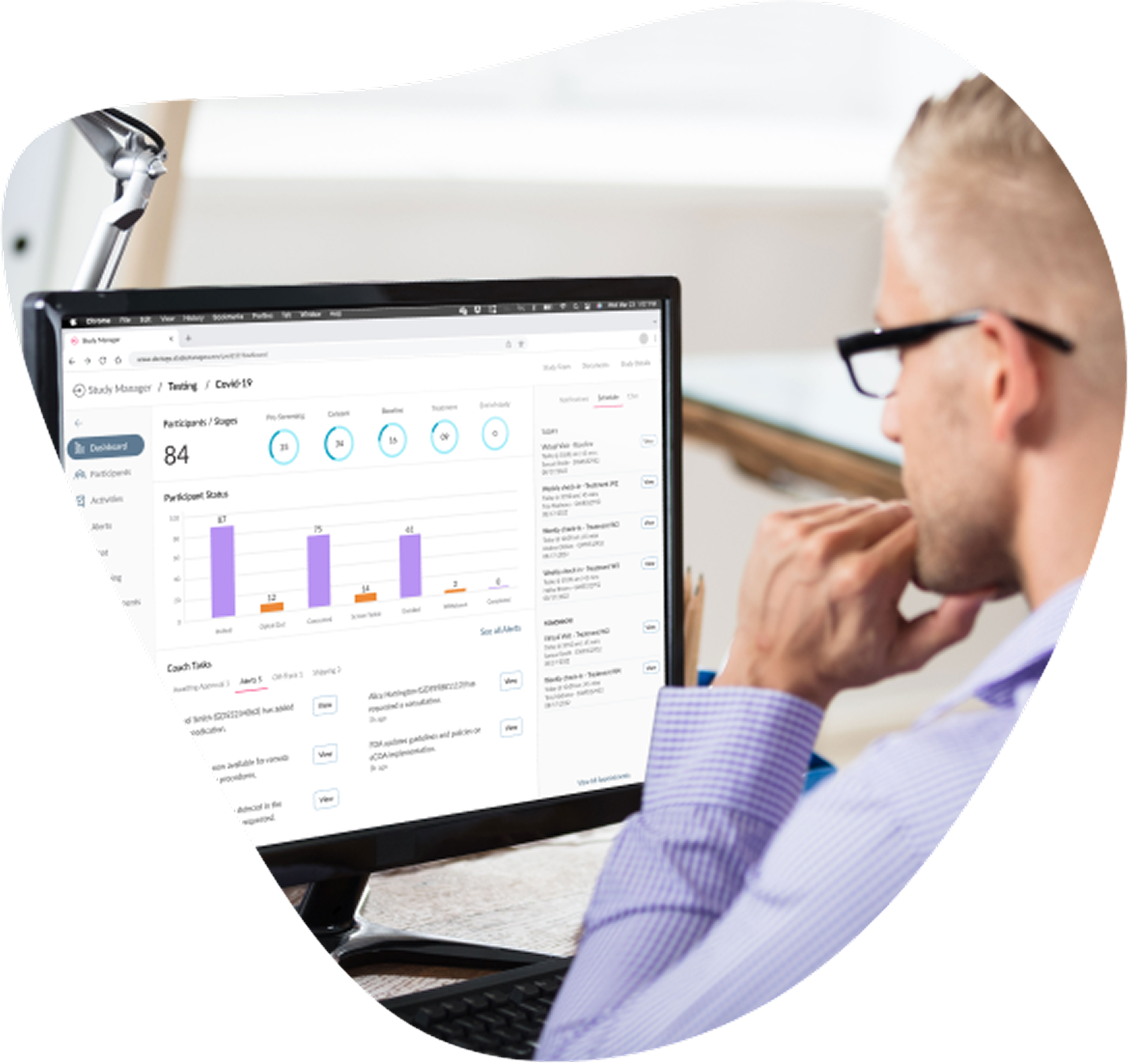
Designed to bring structure and speed to every phase of your trial, Study Manager combines clarity, automation, and oversight in a single solution built for modern clinical teams.


From startup to closeout, Study Manager gives sponsors, CROs, and sites real-time control over trial execution. Teams can monitor progress, manage tasks, track compliance, and make adjustments on the fly—all from one intuitive dashboard. With smart alerts, flexible workflows, and audit-ready logs, Study Manager helps you run smarter trials, resolve issues faster, and scale with confidence.
Track recruitment, ePRO status, protocol deviations, and site activity—all in one centralized workspace. Stay ahead of issues with real-time insights.
Eliminate platform fatigue with a single solution. Study Manager consolidates CTMS, trackers, and spreadsheets into one unified workspace.
Never lose track of a patient or protocol step. Intelligent filters and proactive alerts flag risks early—empowering you to act fast and stay on track.

“ObvioHealth’s platform is really powerful in providing safety oversight. The real-time alerts enabled me to review vaccine data daily to report to senior management. The team was professional and very responsive to requests.”
— Director of Clinical Operations, Biotech Company



Study Manager supports flexible configuration across sites with unique operational needs. Teams can assign languages and documents per site, manage mid-study changes without disrupting active participants, and control how updates are deployed—whether globally or site-specific.
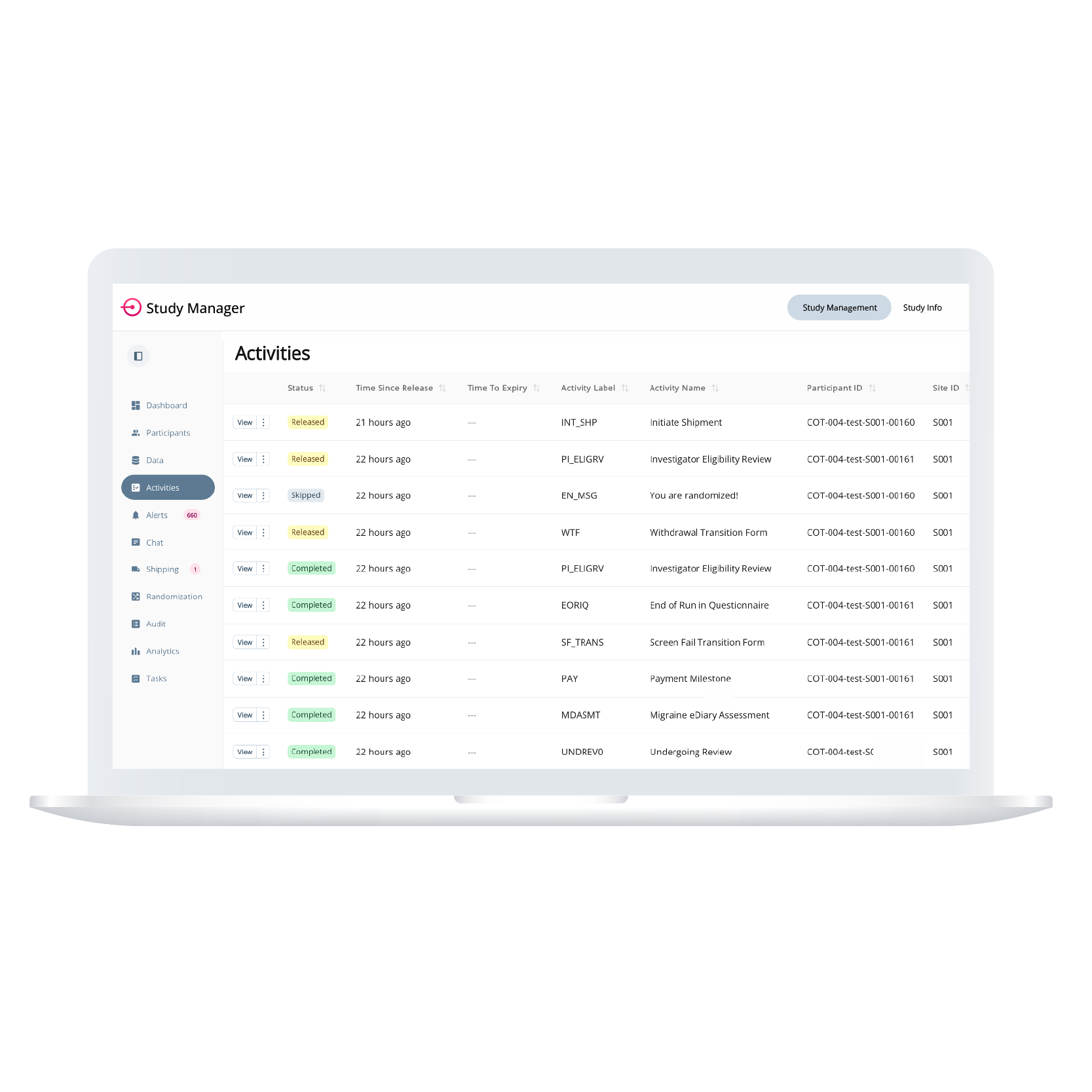
The Centralized Activity Overview provides a centralized view of all study activities across sites and protocol versions. Users can sort and filter by compliance status, time to expiry, or version number to spot issues quickly. Non-compliant or at-risk participants are flagged in real time, with direct navigation into participant records for faster resolution and follow-up.
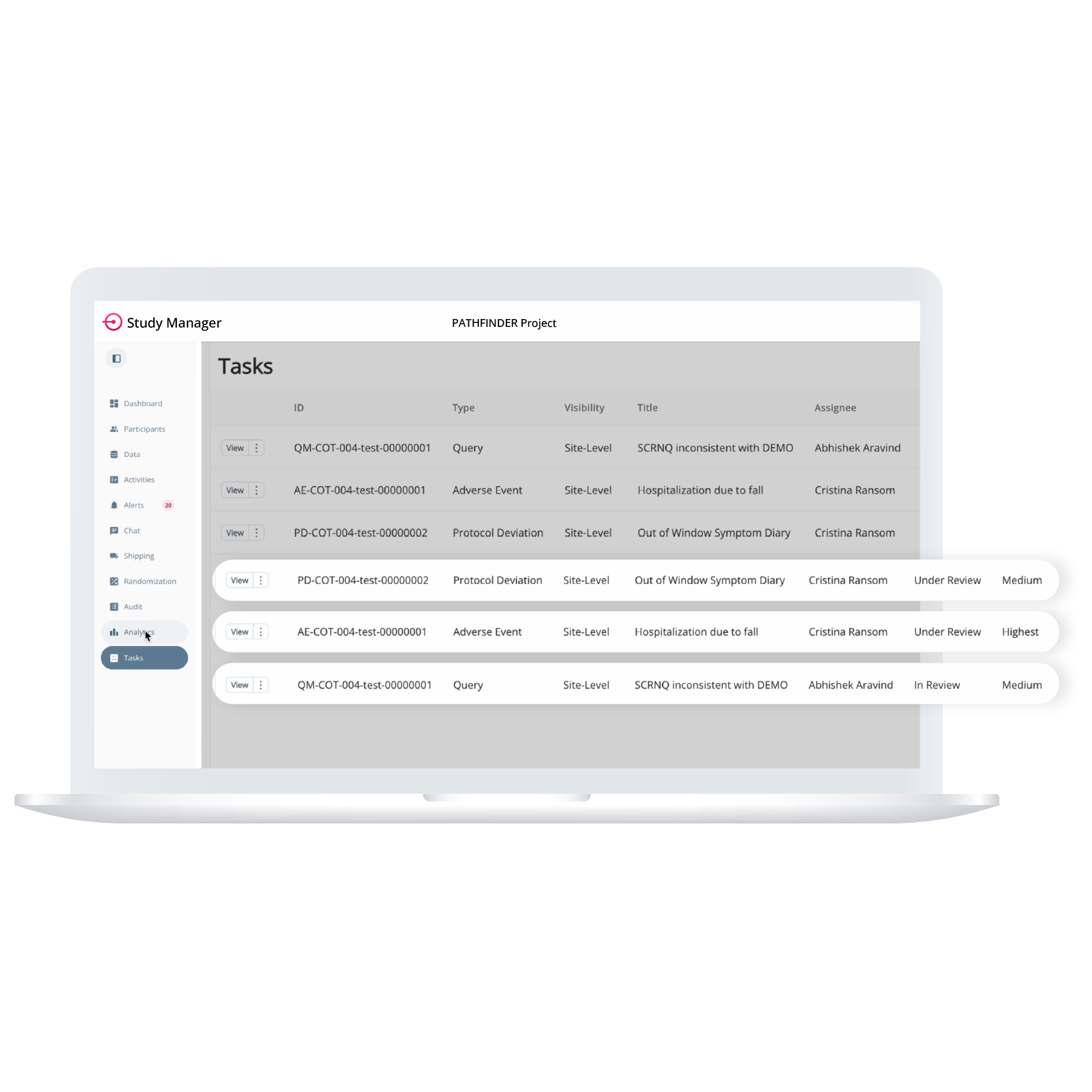
Task Management allows teams to build operational workflows by creating and assigning tasks for protocol deviations, adverse events, queries, or study-specific needs. Tasks can include fields like priority, tags, due dates, and associated participants or activities. Visibility can be controlled at the site level, and the full task history is automatically logged with timestamps to ensure a clear audit trail.
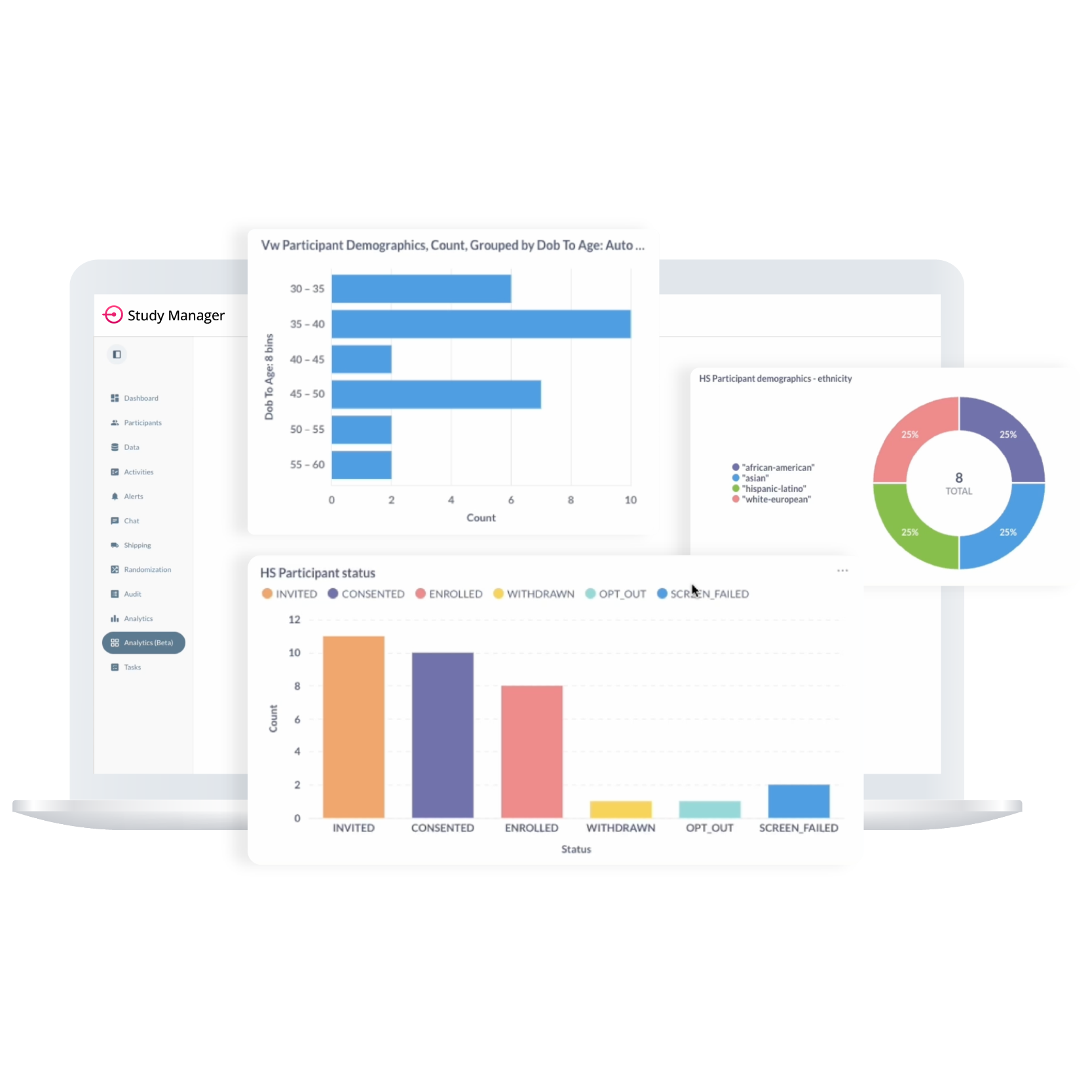
Our analytics dashboards provide real-time visibility into key performance indicators such as compliance trends, activity completion, and screen failure rates. Dashboards are customizable with permission-controlled access and allow direct drill-downs into associated participant and task records. Our engineering team can assist with setup and ongoing configuration to meet your unique reporting needs.
Ensure complete and compliant data capture — even beyond scheduled visit windows. The Study Manager automatically flags expired or out-of-window activities, enabling study teams to review, document, and reinstate them when protocol allows. This flexibility prevents data loss, preserves audit integrity, and ensures every participant contribution is captured.




«Sabíamos que necesitábamos facilitar el estudio a nuestros pacientes. Teníamos el estrés adicional de iniciar este ensayo en el punto álgido de la pandemia. El sitio virtual de ObvioHealth brindó el apoyo que necesitábamos para nuestros pacientes, y su equipo de ciencia clínica nos brindó su experiencia sin la rigidez de un CRO grande».

