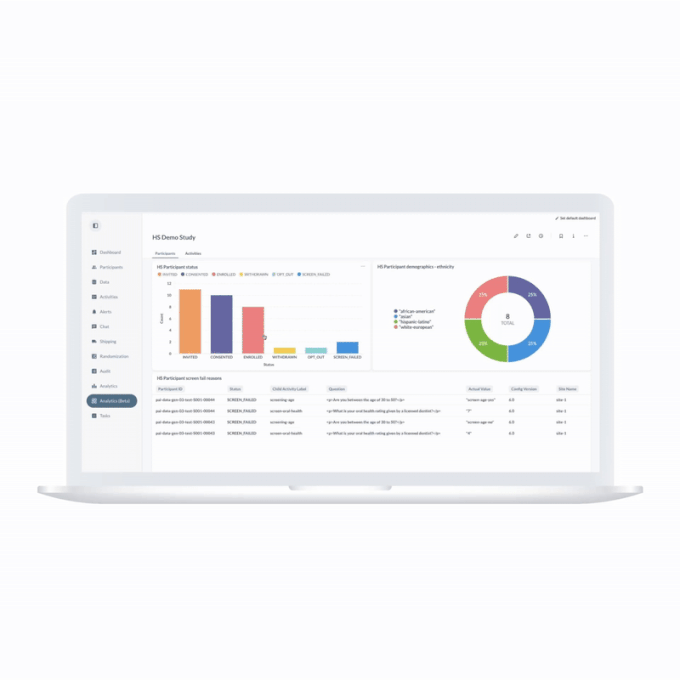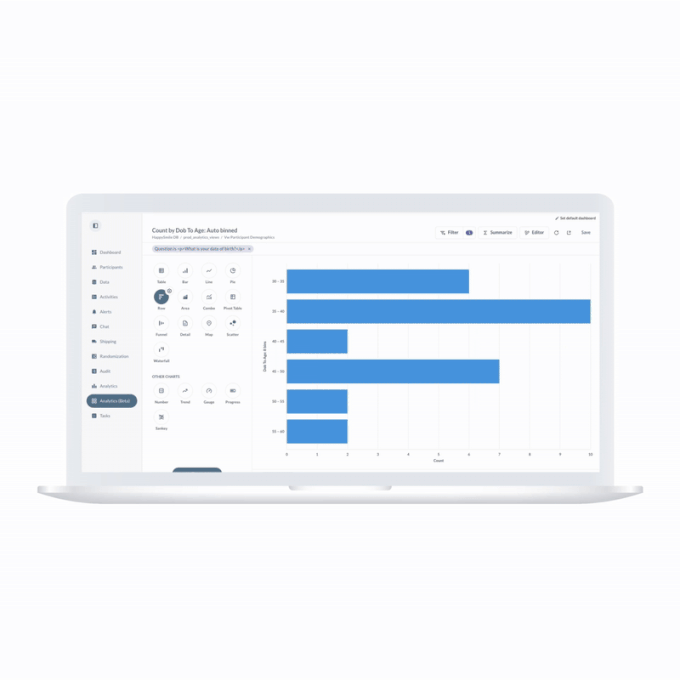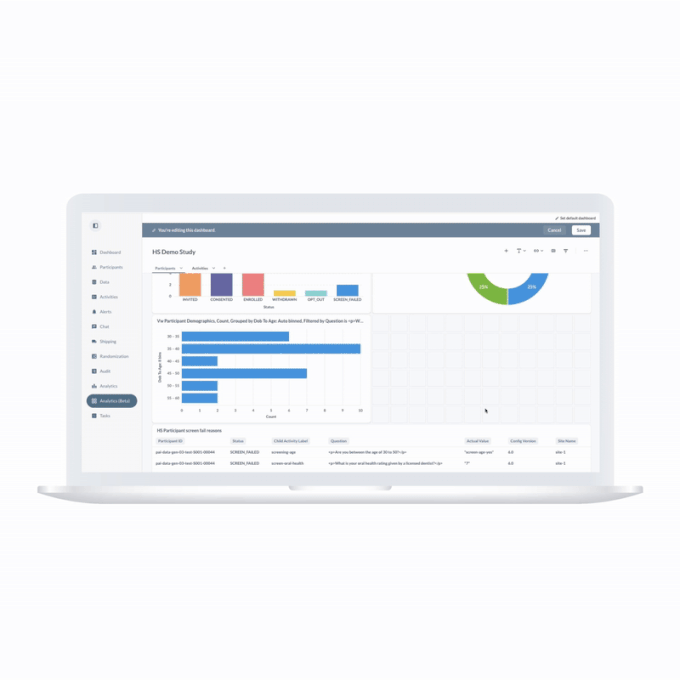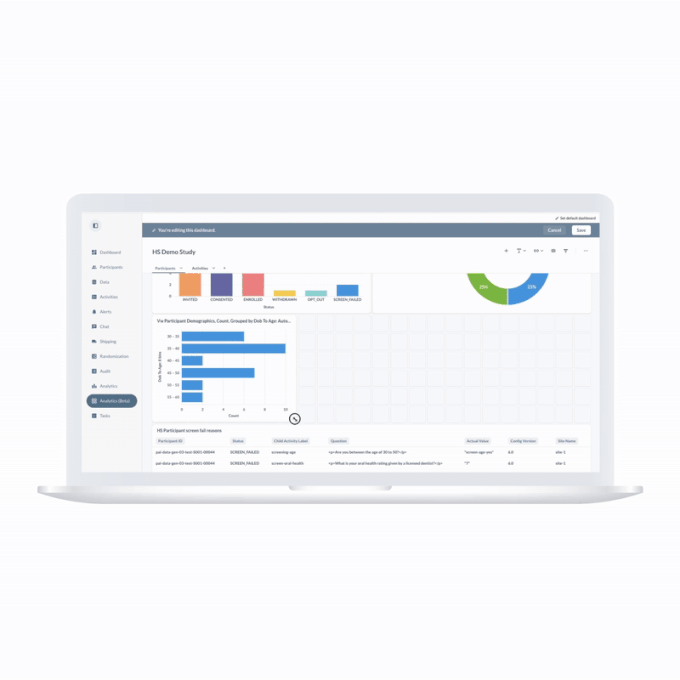
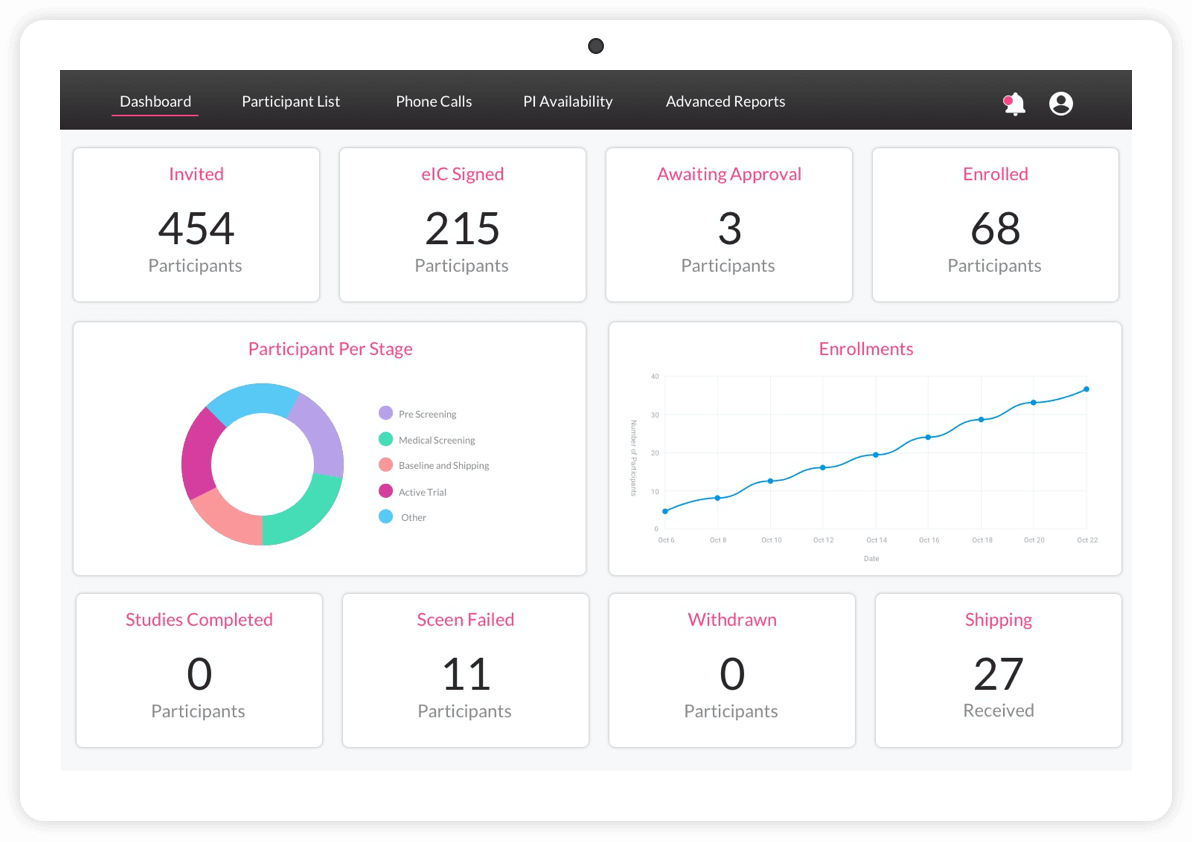
Los ensayos clínicos digitales ofrecen pruebas más sólidas
ObvioHealth es una compañía global de ensayos clínicos digitales que acelera la entrega de pruebas más sólidas. Diseñamos, implementamos y entregamos ensayos clínicos digitales con excelencia operativa. El resultado son datos más precisos para los patrocinadores de los ensayos y una mejor experiencia tanto para los participantes como para los centros.
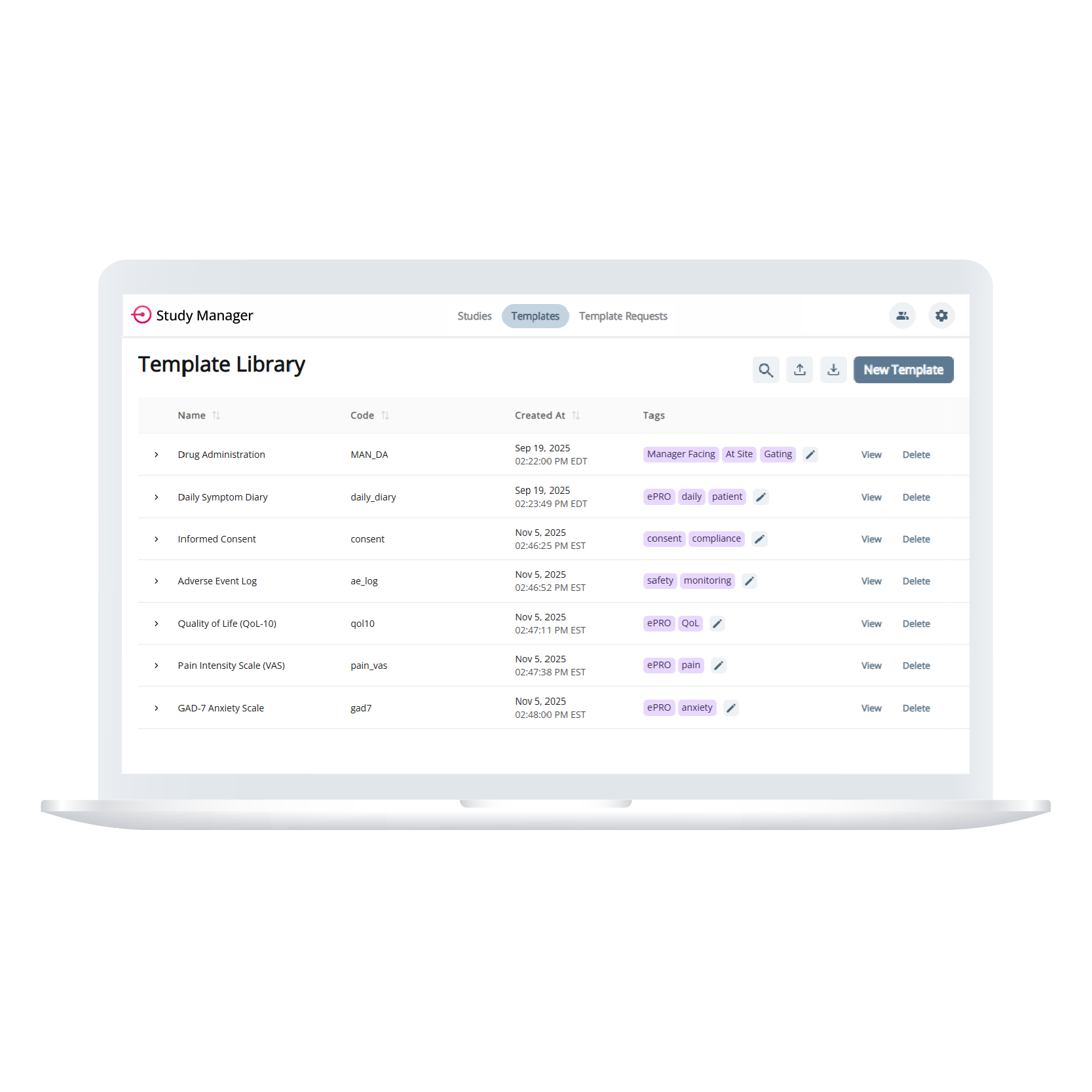
Template Library
























.jpg)