Study Designer
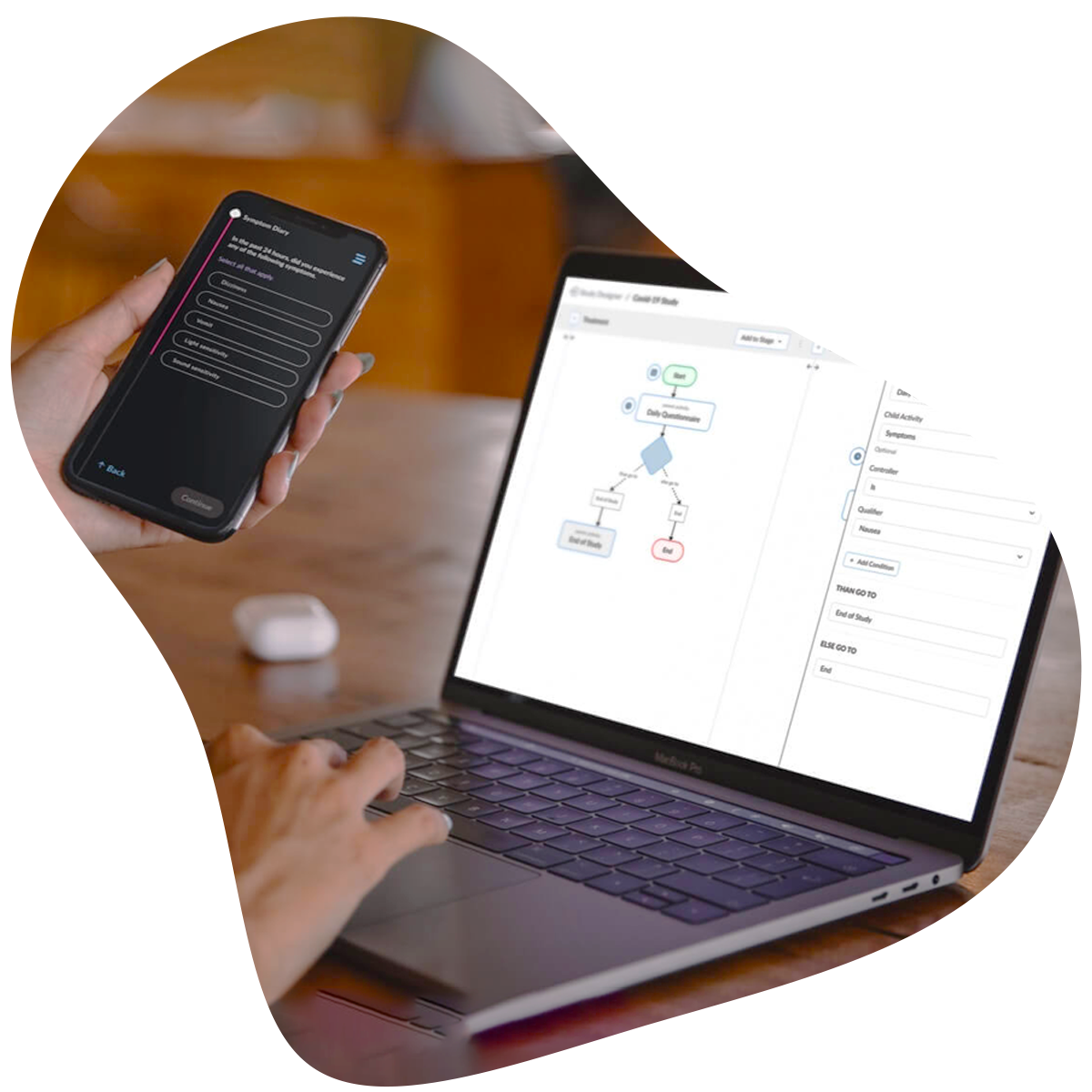
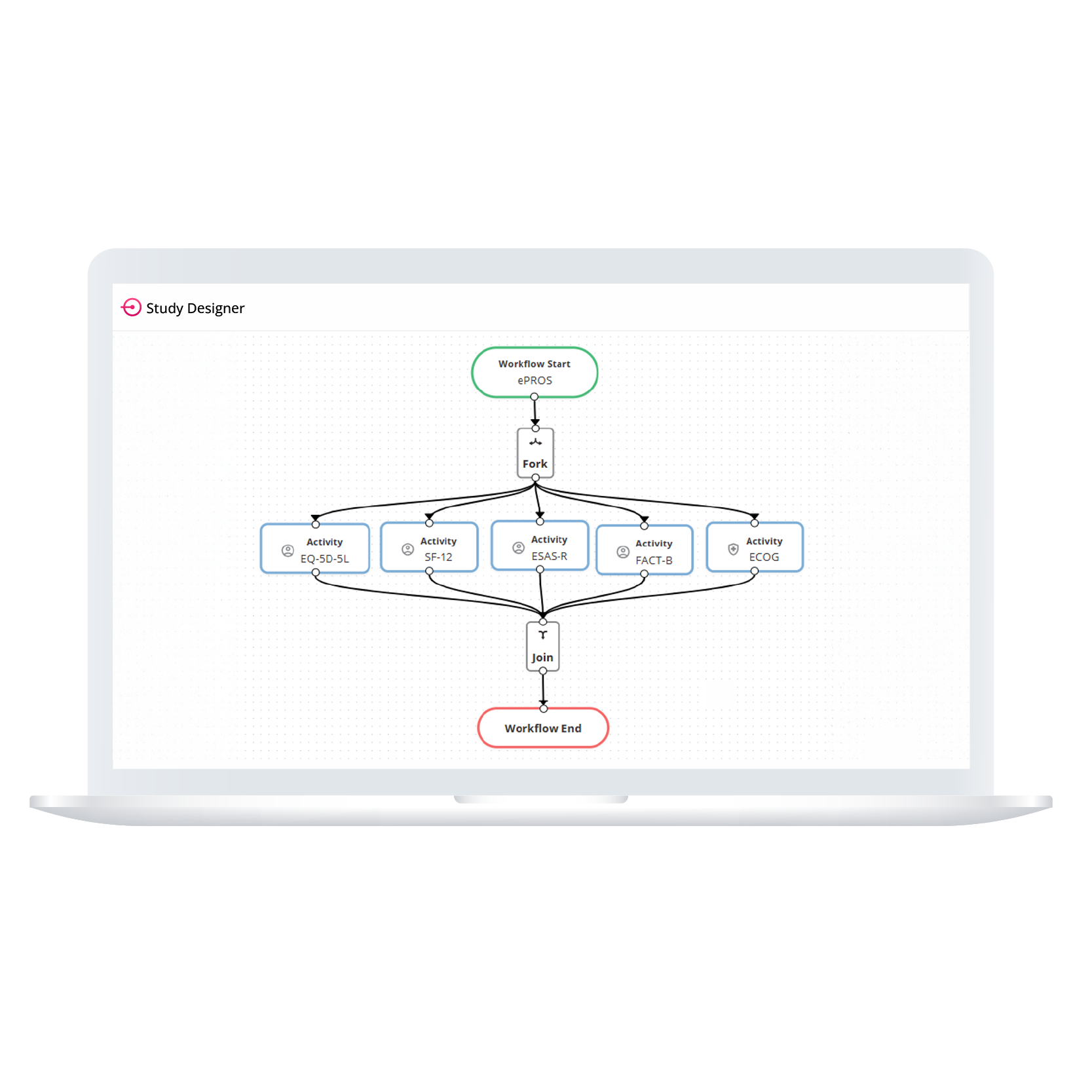
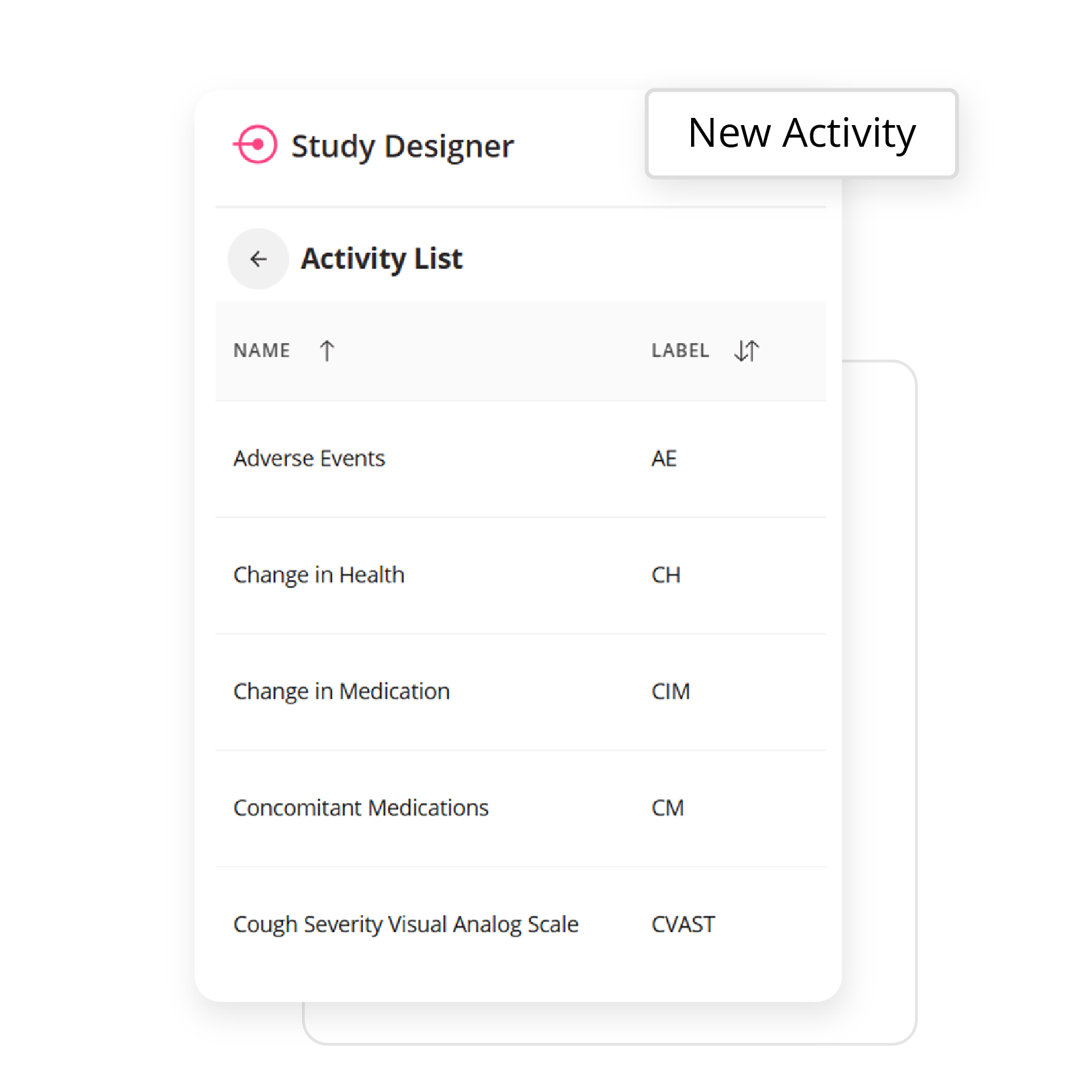
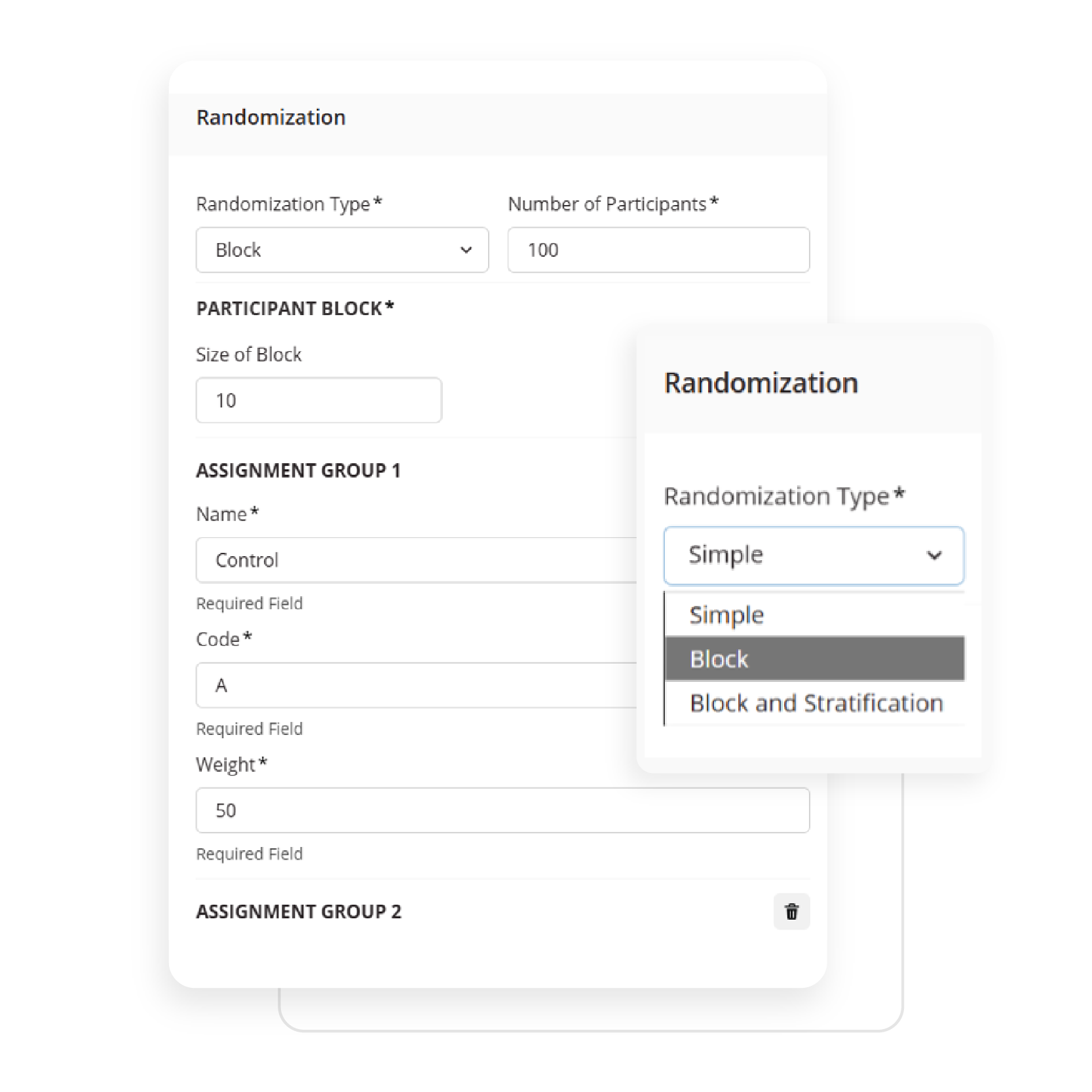
Empower your team to build study workflows that match your protocol exactly. Whether you’re designing an eDiary or managing complex visit windows, ObvioGo Designer gives you drag-and-drop tools to configure logic, set up activities, and manage transitions—all without writing a single line of code.